Industrial ammonia production emits more CO2 than any other chemical-making reaction
Making ammonia with water and nitrogen
New approach uses samarium compound to weaken water’s bonds
by Bethany Halford
APRIL 26, 2019 | APPEARED IN VOLUME 97, ISSUE 17

Credit: ShutterstockThe energy-hungry Haber-Bosch process is used to make ammonia at large scale.
One of the world’s most important industrial chemical processes helps feed humanity, but it also gobbles up energy and generates a significant amount of carbon dioxide. The Haber-Bosch process is used to make ammonia, the raw material for nitrogen-based fertilizers. It combines N2 from air and hydrogen gas using a catalyst, temperatures above 400 °C, and pressures around 40,000 kPa. Seeking a milder method for making NH3, University of Tokyo chemists led by Yoshiaki Nishibayashi found they could use water or alcohols instead of H2 as a source of H for the reduction of N2. Using samarium diiodide (SmI2), they weakened the water or alcohol’s O–H bonds so that they provided H atoms that reacted with N2 in the presence of a molybdenum catalyst to make NH3 (Nature 2019, DOI: 10.1038/s41586-019-1134-2). Not only does this reaction take place at ambient temperature and pressure, but it is also fast—each catalyst molecule produces more than 100 molecules of NH3 per minute. Currently, the reaction isn’t suitable for industrial-scale production of NH3: the large quantities of SmI2 used generate a lot of waste, and it’s not trivial to separate the NH3 from the solution in which it’s made. However, Nishibayashi and colleagues say the work presents a new direction for chemists to take when trying to make greener NH3.Chemical & Engineering NewsISSN 0009-2347Copyright © 2020 American Chemical Society
YOU MIGHT ALSO LIKE…

INORGANIC CHEMISTRYBa-Ce-based perovskite makes ammonia under mild conditions
SYNTHESISNew catalyst transforms carbon dioxide into commodity chemicals
SYNTHESISFunctionalized Biaryls By Organocatalysis
Industrial ammonia production emits more CO2 than any other chemical-making reaction. Chemists want to change that
Scientists around the world are working to reduce how much greenhouse gas the ammonia-making process emits
by Leigh Krietsch Boerner, special to C&EN
JUNE 15, 2019 | APPEARED IN VOLUME 97, ISSUE 24
ADVERTISEMENT
MOST POPULAR IN ENVIRONMENT
- Carbon capture drives metal purification
- EPA adds 160 PFAS to Toxics Release Inventory
- ‘Forever chemicals’ no more? These technologies aim to destroy PFAS in water
- Plastic has a problem; is chemical recycling the solution?
- Plastics recycling with microbes and worms is further away than people think
- Chemistry may have solutions to our plastic trash problem

Credit: SiemensThe Siemens green ammonia test plant uses wind power to convert hydrogen and nitrogen to ammonia.
The Haber-Bosch process, which converts hydrogen and nitrogen to ammonia, could be one of the most important industrial chemical reactions ever developed. The process made ammonia fertilizer widely available, helping cause a world population boom as yields from agriculture increased rapidly in a short time.
Globally, ammonia production plants made 157.3 million metric tons (t) of the compound in 2010, according to the Institute for Industrial Productivity’s Industrial Efficiency Technology Database. Between 75 and 90% of this ammonia goes toward making fertilizer, and about 50% of the world’s food production relies on ammonia fertilizer.
Related: Making ammonia with water and nitrogen
The rest of the ammonia helps make pharmaceuticals, plastics, textiles, explosives, and other chemicals. Almost every synthetic product we use containing nitrogen atoms comes to us through the Haber-Bosch process in some way, says Karthish Manthiram, a chemical engineer from the Massachusetts Institute of Technology. “All those nitrogen atoms came from ammonia, which means that there is this enormous carbon dioxide footprint embedded in all the different products that we use.”
That massive carbon footprint exists because although the Haber-Bosch process represents a huge technological advancement, it’s always been an energy-hungry one. The reaction, which runs at temperatures around 500 °C and at pressures up to about 20 MPa, sucks up about 1% of the world’s total energy production. It belched up to about 451 million t of CO2 in 2010, according to the Institute for Industrial Productivity. That total accounts for roughly 1% of global annual CO2 emissions, more than any other industrial chemical-making reaction (see page 23).
Ammonia by the numbers
157.3 million:
Metric tons of NH3 produced worldwide in 2010
451 million:
Metric tons of CO2 emitted by NH3 synthesis worldwide in 2010.
~1%:
Percentage of global CO2 emissions that come from NH3 synthesis.
Sources: Institute for Industrial Productivity.
The carbon footprint of ammonia synthesis goes well beyond its energy demands. Hydrogen used for the reaction comes from natural gas, coal, or oil through processes that release CO2. According to a 2013 joint report from the International Energy Agency, the International Council of Chemical Associations, and the Society for Chemical Engineering and Biotechnology, CO2 emissions from hydrogen production account for more than half of those from the entire ammonia production process. In total, from hydrocarbon feedstocks to NH3 synthesis, every NH3 molecule generated releases one molecule of CO2 as a coproduct.
And our hunger for ammonia fertilizer is increasing. According to the Food and Agriculture Organization of the United Nations, nitrogen fertilizer demand is projected to increase from 110 million t in 2015 to almost 119 million t by 2020.
Chemists and engineers across the world are trying to make ammonia synthesis sustainable. Some are working to power the reaction with renewable energy sources and to generate hydrogen without fossil fuels. Others want to find a more efficient reaction than Haber-Bosch to synthesize ammonia. The researchers admit that progress has been slow but worth it.
“Ammonia as it’s produced today for fertilizers is effectively a fossil-fuel product,” says Douglas MacFarlane, an electrochemist from Monash University. “Most of our food comes from fertilizers. Therefore, our food is effectively a fossil-fuel product. And that’s not sustainable.”
At green ammonia plants around the world, including in Japan, England, Australia, and the US, researchers have been experimenting with using renewable energy and feedstocks to make the valuable chemical on small scales. These companies mostly use the conventional Haber-Bosch process, but instead of relying on fossil fuels to generate hydrogen and power the reactions, they’re using water electrolysis and alternative energy sources.
Since last year, the Japanese company JGC has been trying these approaches at a trial plant at the Fukushima Renewable Energy Institute. Through a national program called the SIP Energy Carriers, the company has teamed up with the National Institute of Advanced Industrial Science and Technology (AIST) to get the green ammonia demonstration plant up and running. It can run on solar power, produces hydrogen through water electrolysis, and operates a Haber-Bosch-type reaction using a new ruthenium catalyst that JGC developed with AIST.
“The major advantage of our process is that hydrogen is produced at a much lower pressure than the conventional process,” says Mototaka Kai, project manager at the plant. The hydrogen pressure is around 5 MPa, Mototaka says, which is around one-third to one-quarter that of a traditional Haber-Bosch plant. This lower pressure has two advantages. The reaction is safer because it’s operating at a lower pressure. Plus, the plant requires less energy to pressurize the system. Currently, the plant produces 20–50 kg of ammonia per day.
Siemens in the UK is working with researchers at the University of Oxford, the UK’s Science and Technology Facilities Council, and Cardiff University to run a demonstration plant using the typical Haber-Bosch process, powering it with wind. Ian Wilkinson, program manager in corporate technology at Siemens, names two reasons the firm chose to use only mature technology available today to run its plant. First, Siemens wants to show that it can produce ammonia renewably, in a way that it can quickly scale up. The company also views the plant as a test system for ongoing technology development, including Haber-Bosch catalyst development and ammonia combustion tests.
The plan has worked so far. The small plant, set up in shipping containers, takes electricity from a wind turbine, runs it through a hydrogen electrolysis unit, and then uses the resulting hydrogen to synthesize ammonia. If the company runs the plant continuously, it gets 30 kg of ammonia a day, Wilkinson says. “It’s a small-scale, proof-of-principle system,” he says, noting that the only thing in the plant that the firm didn’t buy off the shelf is the synthesis loop in which the actual Haber-Bosch reaction takes place. “We had to build our own. You can’t buy them this small,” he says.
Ammonia synthesis at a wind farm could help solve one of the biggest problems with renewable energy sources—they produce energy intermittently. The sun doesn’t always shine and the wind doesn’t always blow, so how do you generate electricity consistently? Burning ammonia produced renewably may be one answer, Wilkinson says. Both Siemens and JGC are interested in green ammonia production not just to make fertilizer but also to synthesize a carbon-free fuel. Similar to gasoline, ammonia can be shipped and stored, and it is easier to deal with than gaseous hydrogen, another possible carbon-free fuel.
“Ammonia is what I like to call a nexus molecule,” Manthiram says. “It’s useful as a fertilizer. It’s useful for food. It’s useful for energy storage.” Electricity generated through renewable sources can combine nitrogen from the air and hydrogen from water to make a transportable fuel, he says. And companies already ship ammonia across oceans for current uses, MacFarlane says. “That technology is well understood in large quantities of ammonia.”
Related: Green method makes methanesulfonic acid from methane
But no matter how these companies plan to use the ammonia produced by their green plants, they’re still mostly using Haber-Bosch to synthesize the molecule. The reaction involves combining hydrogen and nitrogen gas over an iron catalyst, at high temperatures and pressures. And it isn’t efficient, MacFarlane says. Each metric ton of ammonia packs about 5 MW h of energy. “The best, most efficient Haber-Bosch plants work at around 10 MW h per metric ton of ammonia,” MacFarlane says. “So we’re approximately only 50% efficient. It’s wasting a lot of energy for what you get.”
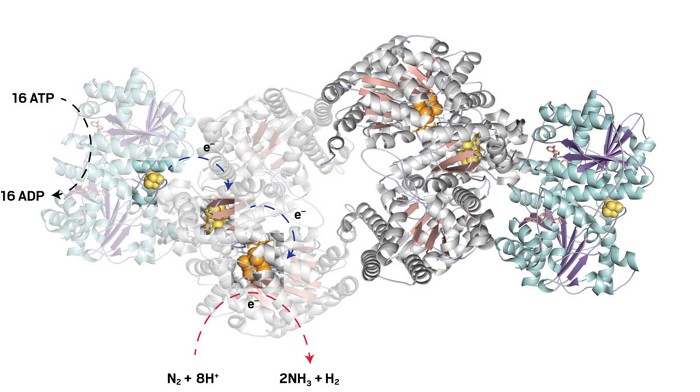
Credit: Rong CaiNitrogenase is the only enzyme known to reduce N2 to NH3 at ambient temperature and pressure. The enzyme breaks down the energy-rich adenosine triphosphate (ATP) to adenosine diphosphate (ADP) and uses that energy to power the transfer of electrons from an iron-sulfur cluster (cyan/yellow) through a phosphorus cluster (pink/yellow) to the iron-molybdenum catalytic cofactor (orange), where N2 gets reduced.
Switching to renewable feedstocks and energy sources is a good solution in the short term, Manthiram says, because companies can effectively combine current renewable energy technologies with Haber-Bosch. But to improve the sustainability of ammonia synthesis over the long term, scientists have to change the game entirely.
“Many people are looking at alternatives to Haber-Bosch,” says Shelley Minteer, a bioelectrochemist at the University of Utah. “How can we do something at low temperatures and atmospheric pressure or near atmospheric pressure?”
Research in the field has taken off since about 2015, perhaps because of expanded funding availability as federal agencies have started to focus on the topic, says Lauren Greenlee, a chemical engineer at the University of Arkansas. Researchers are trying a wide range of approaches: electrochemistry, electrocatalysis, photocatalysis, and photoelectrocatalysis. And they’re even taking inspiration from biochemistry. “That diversity as a field that’s growing so quickly is actually fantastic because then you’re able to learn from each other what works and what doesn’t,” Minteer says.
Electrochemical reduction of nitrogen to ammonia over a catalyst has captured the imagination of many scientists. The chemists apply a voltage across an electrochemical cell to drive both water oxidation and nitrogen reduction simultaneously. The catalyst at the anode oxidizes water to form hydrogen ions, which migrate to the cathode, where a different catalyst reduces nitrogen to ammonia. Scientists have developed numerous electrochemical ammonia-synthesis catalysts, including noble-metal nanostructures, metal oxides, metal nitrides, metal sulfides, nitrogen- and boron-doped carbon, and lithium metal.
“What’s enticing about [electrochemistry] is that you can get your hydrogen atoms directly from water molecules without having to go through molecular hydrogen,” Greenlee says. “If, in theory, your electrochemical process is being driven by renewable energy, you eliminate the need for fossil fuels both from an energy input standpoint for electricity, but also from a hydrogen production standpoint.” This method also avoids the need to do electrolysis as a separate step and has the potential to operate at low pressure and possibly low temperature, she says. There are “a lot of pieces of it that are really positive, if we can get it to work.”
Electrochemistry also presents a good way to solve a trade-off between reaction rates and yields that chemists must face when running the Haber-Bosch reaction, Manthiram says. The reaction has good yields at very low temperatures, he says, but the rate is sluggish. To speed it up, chemists raise the temperature. But at those high temperatures, the reaction’s thermodynamics change, and the yield goes down. So chemists raise the pressure to bring the yields back up. What’s special about an electrochemical system is that chemists can increase voltage instead of pressure, Manthiram says. “What normally takes hundreds of bar pressure to achieve can be done with fractions of a volt.”
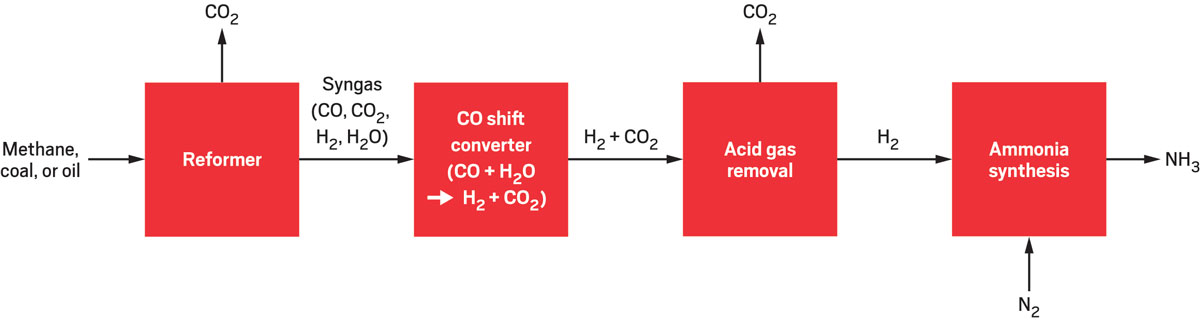
Current process
Today, ammonia synthesis starts with generating hydrogen gas from fossil-fuel feedstocks. A reformer turns the feedstocks into a mixture of gases called synthesis gas (syngas), which includes hydrogen. A CO shift converter combines water and the carbon monoxide from syngas to form CO2 and more hydrogen, and then acid gas removal isolates the hydrogen for ammonia synthesis. This process releases CO2 at various steps along the way.
One of the other possible advantages of the electrochemical approach is that the reaction system can be small. A device under development in MacFarlane’s lab is about the size of a cell phone. The idea is that it could synthesize ammonia for fertilizer on the scale of a farm or greenhouse, so the material could be used right where it’s made, eliminating the need for transport, MacFarlane says.
Meanwhile, other researchers are looking to nature to understand how to efficiently reduce nitrogen to ammonia. Some bacteria use large protein complexes called nitrogenases to grab nitrogen out of the air and make ammonia. Minteer and her team have been studying this system to connect these bacterial enzymes to electrodes to create new electrocatalysts. But they still have a long way to go, Minteer says. Their systems do more proton reduction than ammonia production. The goal is to get to the point where they’re making 99% ammonia and 1% hydrogen. Right now, their systems make about 40% ammonia and 60% hydrogen, she says.
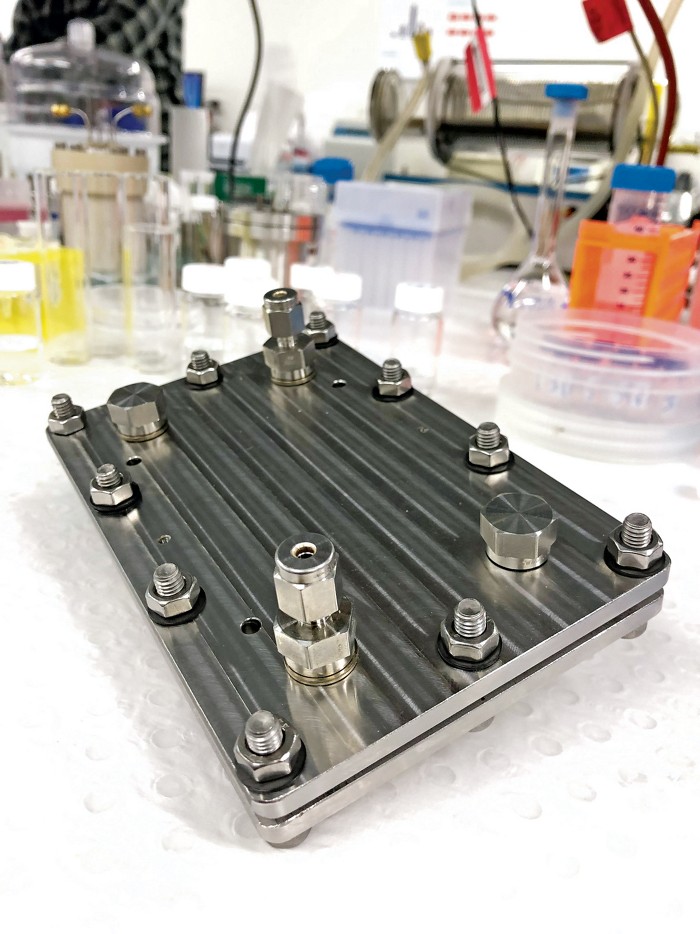
Credit: Douglas MacFarlaneThis device, developed by Douglas MacFarlane and coworkers at Monash University, can convert hydrogen and nitrogen to ammonia inside a cell phone–sized package.
Scientists throughout the field face this problem with catalyst yield and selectivity. As a result, the ammonia coming out of these non-Haber-Bosch systems is a trickle, not a torrent. It’s easier, chemically, to make hydrogen gas than ammonia. “Most catalysts that would be active for nitrogen reduction are also really active for hydrogen evolution,” Greenlee says.
“The catalyst needs to be able to break the nitrogen triple bond, which is a very strong and inert bond,” Manthiram says. Once the bond breaks, the catalyst needs to form the three nitrogen-hydrogen bonds, all at ambient conditions without high temperatures to accelerate the kinetics. That’s a tall order and something that hasn’t yet been accomplished, he says.
But maybe chemists have accomplished it and just didn’t notice, Minteer says. Scientists have been intensely studying hydrogen-evolution catalysts for about the past 20 years. “We’ve learned a lot about how to make good hydrogen-evolution catalysts.” It’s possible, she thinks, that some of the catalysts that failed at hydrogen evolution were actually good at making ammonia. “We need to essentially learn about all those catalysts that people made that didn’t work, that don’t produce hydrogen, and see if they are useful for producing ammonia,” she says.
ADVERTISEMENT
Greenlee points out that the solutions have to go beyond catalyst design. Scientists need to figure out how to control, reduce, or eliminate the hydrogen-evolution reaction. “It’s going to be some combination of catalyst design and controlling the surface environment of the catalyst or the interface to be able to control or suppress hydrogen,” she says.
Besides this selectivity issue, scientists also have to worry about.
how long these catalysts last, MacFarlane says, and it’s something that many groups are not thinking about yet. For a new ammonia production system to be practical, such as in an electrochemical device like the one his group is working on, catalysts will need to remain active and viable for years, even if the system could be taken apart and refurbished, he says. “Catalyst lifetime is a challenge that’s yet to be clearly identified and understood.” Most people are not publishing data on lifetimes, but the longest he’s seen is about a day, he says.
Related: Tackling sustainable fertilizer production with an alternative electrolyte
The road to Haber-Bosch-free ammonia is long, Minteer says. Whether it’s an electrocatalysis, photocatalysis, or biocatalysis system, any promising lab-scale reaction will still take at least a decade or two to make commercial scale, she says.
Searching for alternatives to Haber-Bosch is also risky, Manthiram says, because what scientists are pursuing now may not pan out. But with ammonia production touching so many things that we use every day, including our food and pharmaceuticals, scientists need to find a way to make these lab-scale systems work on larger scales, he says. “It’s hard to imagine a world where we’re just going to be OK with the way that we make ammonia today.”
Errors from the air: The trials and tribulations of developing ammonia catalysts
When Shelley Minteer at the University of Utah first got started studying how bacterial enzymes called nitrogenases produce ammonia, she noticed something funny. Some days, the complexes wouldn’t produce ammonia. On other days, they’d produce a lot. The culprit? The cleaning lady.
“We would see spikes in production the days she cleaned the floor,” Minteer says.
Nitrogen and ammonia are all around us. Nitrogen makes up 78% of the air we breathe, and nitrogen-containing molecules like ammonia are in numerous plastics, textiles—and cleaning supplies. These molecules can stick to tubing, gloves, and glassware. “It‘s very difficult, if not impossible, to get all of the contaminants of ammonia out of all the samples,” Minteer says. Contaminants also include other nitrogen-containing compounds, such as nitrites and nitrates, which can easily react to make ammonia.
The field of new ammonia-producing catalysts is still young, says Lauren Greenlee, a chemical engineer at the University of Arkansas. “The catalysts just are not very efficient.” Scientists make small amounts of a catalyst and then test it in small-scale setups. “The problem is that the amounts of ammonia that are actually produced by many electrocatalysts are not much larger than what you might measure in the background.”
So how do you know if the ammonia you’re measuring actually came from your catalyst instead of from contaminants in the lab? Without proper controls, you don’t, Minteer says. If part of that ammonia is coming from the background, scientists might think that their catalyst is working well when it may not be.
Currently, journals don’t require data on specific control experiments to publish data from an ammonia-producing catalyst. Whether the journals should require those controls is a matter of debate in the community. “I’ve talked to some people who have argued that we should wait and not do controls,” Greenlee says. Maybe, these members of the field argue, the catalyst community will move forward, and catalysts will get more efficient so that the difference between what the catalyst is producing and the amount of ambient ammonia will become larger.
While that may happen, that wait-and-see approach has issues, Greenlee says. If a group reports a high-performing catalyst, other researchers may start working with it, thinking that it’s an improvement. “But what if that’s not the right direction to go because the group didn’t measure their background correctly?” Greenlee asks.
Greenlee thinks that researchers should run controls and take background measurements for every catalyst on every day they run experiments. Such controls would include running experiments with isotopically labeled molecules as a final evaluation of successful catalysts so scientists know where the nitrogen in ammonia came from. Papers should also report the results from these control experiments. “Even if a lab is doing appropriate controls, it’s very hard to tell as a reviewer” because they’re not adequately reported in the paper, she says.
“There are surely errors made in the history of science,” says Karthish Manthiram, a chemical engineer at the Massachusetts Institute of Technology. “As long as everyone admits to their errors, we all move forward together.”
Additional report
| THE HABER PROCESSThis page describes the Haber Process for the manufacture of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. It looks at the effect of temperature, pressure and catalyst on the composition of the equilibrium mixture, the rate of the reaction and the economics of the process. | |
| Important: If you aren’t sure about using Le Chatelier’s Principle or about the effect of changing conditions on rates of reaction you should explore these links before you go on.When you are reading this page, if you find that you aren’t understanding the effect of changing one of the conditions on the position of equilibrium or on the rate of the reaction, come back and follow up these links. | |
| A brief summary of the Haber ProcessThe Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The reaction is reversible and the production of ammonia is exothermic.A flow scheme for the Haber Process looks like this:Some notes on the conditionsThe catalystThe catalyst is actually slightly more complicated than pure iron. It has potassium hydroxide added to it as a promoter – a substance that increases its efficiency.The pressureThe pressure varies from one manufacturing plant to another, but is always high. You can’t go far wrong in an exam quoting 200 atmospheres.RecyclingAt each pass of the gases through the reactor, only about 15% of the nitrogen and hydrogen converts to ammonia. (This figure also varies from plant to plant.) By continual recycling of the unreacted nitrogen and hydrogen, the overall conversion is about 98%.Explaining the conditionsThe proportions of nitrogen and hydrogenThe mixture of nitrogen and hydrogen going into the reactor is in the ratio of 1 volume of nitrogen to 3 volumes of hydrogen.Avogadro’s Law says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. That means that the gases are going into the reactor in the ratio of 1 molecule of nitrogen to 3 of hydrogen.That is the proportion demanded by the equation.In some reactions you might choose to use an excess of one of the reactants. You would do this if it is particularly important to use up as much as possible of the other reactant – if, for example, it was much more expensive. That doesn’t apply in this case.There is always a down-side to using anything other than the equation proportions. If you have an excess of one reactant there will be molecules passing through the reactor which can’t possibly react because there isn’t anything for them to react with. This wastes reactor space – particularly space on the surface of the catalyst.The temperatureEquilibrium considerationsYou need to shift the position of the equilibrium as far as possible to the right in order to produce the maximum possible amount of ammonia in the equilibrium mixture.The forward reaction (the production of ammonia) is exothermic.According to Le Chatelier’s Principle, this will be favoured if you lower the temperature. The system will respond by moving the position of equilibrium to counteract this – in other words by producing more heat.In order to get as much ammonia as possible in the equilibrium mixture, you need as low a temperature as possible. However, 400 – 450°C isn’t a low temperature!Rate considerationsThe lower the temperature you use, the slower the reaction becomes. A manufacturer is trying to produce as much ammonia as possible per day. It makes no sense to try to achieve an equilibrium mixture which contains a very high proportion of ammonia if it takes several years for the reaction to reach that equilibrium.You need the gases to reach equilibrium within the very short time that they will be in contact with the catalyst in the reactor.The compromise400 – 450°C is a compromise temperature producing a reasonably high proportion of ammonia in the equilibrium mixture (even if it is only 15%), but in a very short time.The pressureEquilibrium considerationsNotice that there are 4 molecules on the left-hand side of the equation, but only 2 on the right.According to Le Chatelier’s Principle, if you increase the pressure the system will respond by favouring the reaction which produces fewer molecules. That will cause the pressure to fall again.In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. 200 atmospheres is a high pressure, but not amazingly high.Rate considerationsIncreasing the pressure brings the molecules closer together. In this particular instance, it will increase their chances of hitting and sticking to the surface of the catalyst where they can react. The higher the pressure the better in terms of the rate of a gas reaction.Economic considerationsVery high pressures are very expensive to produce on two counts.You have to build extremely strong pipes and containment vessels to withstand the very high pressure. That increases your capital costs when the plant is built.High pressures cost a lot to produce and maintain. That means that the running costs of your plant are very high.The compromise200 atmospheres is a compromise pressure chosen on economic grounds. If the pressure used is too high, the cost of generating it exceeds the price you can get for the extra ammonia produced.The catalystEquilibrium considerationsThe catalyst has no effect whatsoever on the position of the equilibrium. Adding a catalyst doesn’t produce any greater percentage of ammonia in the equilibrium mixture. Its only function is to speed up the reaction.Rate considerationsIn the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor.Separating the ammoniaWhen the gases leave the reactor they are hot and at a very high pressure. Ammonia is easily liquefied under pressure as long as it isn’t too hot, and so the temperature of the mixture is lowered enough for the ammonia to turn to a liquid. The nitrogen and hydrogen remain as gases even under these high pressures, and can be recycled.Questions to test your understandingIf this is the first set of questions you have done, please read the introductory page before you start. You will need to use the BACK BUTTON on your browser to come back here afterwards.questions on the Haber ProcessanswersWhere would you like to go now?To the equilibrium menu . . .To the Physical Chemistry menu . . .To Main Menu . . . |